Dubai: When the polio vaccine was first approved (1955), people reportedly ran out to celebrate and church bells rang. Poliomyelitis, the virus that had disfigured millions and afflicted humanity for centuries, finally found its match.
Today, December 2, 2020, the first COVID-19 vaccine using the mRNA technology was approved in the UK, the first government to do so. It’s a moment the world had been waiting for. And it finally came.
The announcement is significant. “The Government has today accepted the recommendation from the independent Medicines and Healthcare products Regulatory Agency (MHRA) to approve Pfizer-BioNTechs COVID-19 vaccine for use,” the British government said. “The vaccine will be made available across the UK from next week.”
Why is this move significant?
It’s a moment of celebration. Markets had already been doing so for the last two weeks, in anticipation of the approval. There are signs aplenty that this day would come, though most didn’t know when. COVID had changed everything we know about life and death, and about vaccines. London said it had approved the Pfizer-BioNTech vaccine under an emergency-user authorisation (EUA). In brief, the move signifies the following:
- It’s the COVID-19 vaccine to be approved for emergency use in a Western country.
- The first government approval for mRNA, a revolutionary and new class of vaccines.
- The first mRNA vaccine to be developed in less than 1 year.
More important, it’s seen as only the first of a number of similar moves that could change the science behind vaccinology itself.
Milestone
It is, without a doubt, a milestone for medicine. Immunisation is a mix of science and miracle. For more than 200 years, the technology used to make vaccines had been the same: get a version of the pathogen, manipulate it in a lab enough to “inactivate” or “attenuate”, and inject the dose into the human body to induce an immune response. In essence, the process trains the human body to heal itself.
mRNA changes a major part of that process, in the substance that gets injected into the human body. It’s a new way (or “platform”) of developing immunisation shots, without actually using a pathogen. Instead, it uses a lab-created genetic sequence that’s turned into a “messenger”, a process that started to see development in the late 1980s.
But while this potential had been proven in the lab, it has never been tested in clinicial trials. In theory, it’s the most advanced method in medical therapy. But none of the mRNA-based solutions proposed to deal with different diseases had received any government approval.
Until the British government greenlight came, this relatively platform remained unproven. Now it promises to revolutionise vaccine development, going forward.
Leading edge
Until the the Phase-3 trial results came out, mRNA only held a promise. But its advantages are well known. First advantage: It is well-suited to rapid adaptation. The UK government authorisation provides a remarkable proof that this fascinating new type of vaccine could actually work. And it’s been proven in extensive trials involving up to 40,000 volunters for Pfizer and 30,000 for Moderna.
There’s a good reason that these shots came out of the labs into the clinical trial arena: They’re relatively much faster to reproduce compared to traditional vaccines.
mRNA technology harnesses genetic engineering. Without the ability to sequence and manipulate genes, this would have been impossible to do in the past, before the time of super-fast computers. Today, mRNA a sort of the iPhone of vaccinology — the platform is the operating system, and the disease-specific vaccine is the app. SARs-CoV-2 could then be just one of the mRNA apps.
Advantages of mRNA
Messenger RNA instructs cells to produce many substances that allow the body to function. These vaccines use carefully-designed mRNA strands to teach cells to create a modified version of a specific coronavirus protein, the so-called “S” (spike) protein or “exo-protein” found on the viral surface of SARS-CoV-2. Once that messenger RNA is injected into the body, it prompts an immune response that can kill the real virus when it comes.
In essence, this process turns cells into tiny drug factories. This is a huge deal, that was never there in the past. Traditional vaccines use segments of viruses or entire viruses (virions) that are killed (“inactivated”) or weakened (“attenuated”). These are tried-and-true processes, often highly effective, but they’re by nature time-consuming.
with mRNA, drugmakers can change the instructions provided by the shots to address specific diseases. That skips the need for growing big batches of a protein or virus. This also means that, in theory, vaccine researchers could respond more faster and nimbler to possible mutations in the SARS-CoV-2 (which causes COVID-19), should they arise. That makes mRNA vaccines the next-generation platform to respond to new viral threats that have a pandemic potential.
What will happen to Moderna mRNA vaccine?
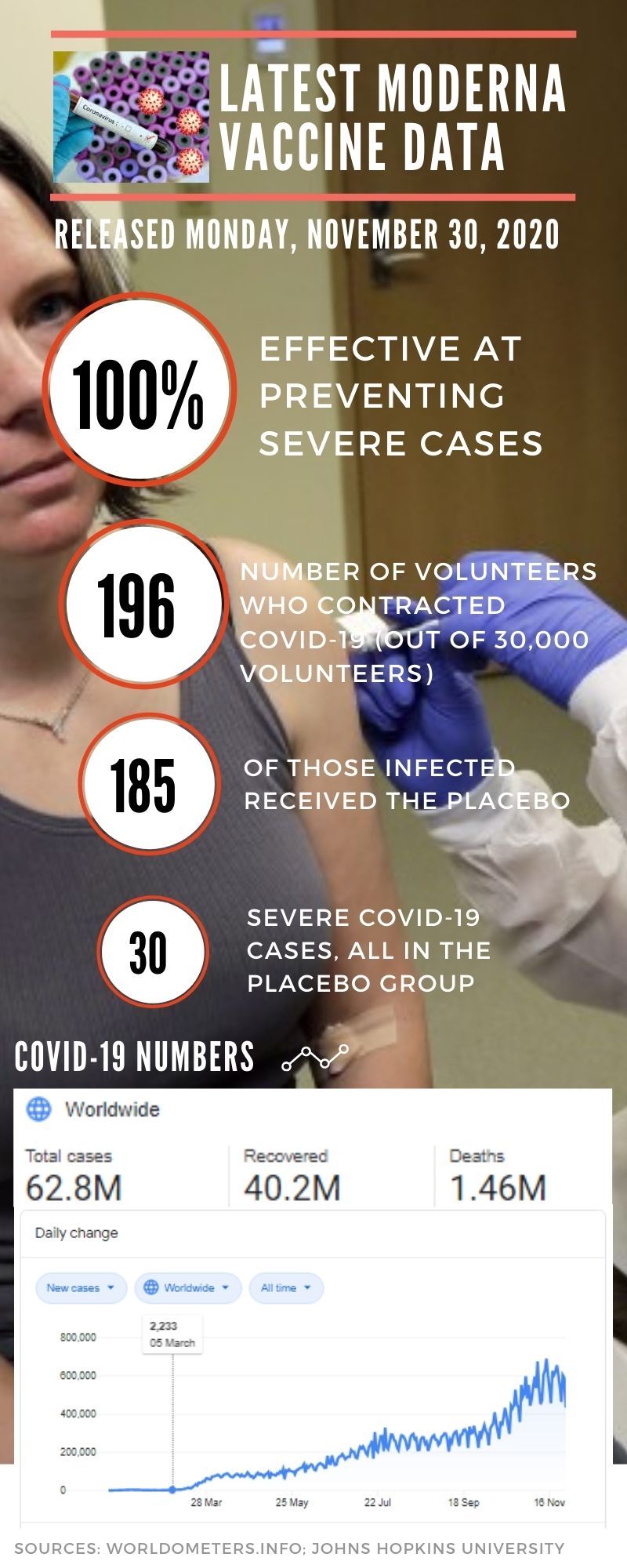
Pfizer released its initial results a week ahead of Moderna. So it’s possible the Western world may have at least two effective vaccines against COVID-19. Moderna’s 30,000-person clinical trial announced a 94.5% efficacy. A subsequent readout on a much bigger number of cases, announced on Monday, shows 94.1% efficacy. Data scienctists said the numbers confirm the previous readout, while keeping the rigorous method of the clinical trial — placebo controlled, randomised and double-blind.
Placebo is a “fake” drug or vaccine — it looks like the real thing but contains nothing therapeutic.
“Double blind” is part of the rigors of clinical trials with a deliberate intention: neither the vaccine administrators nor Moderna CEO Stephan Bancel (or his Pfizer counterpart Albert Bourla) or their people knew who got the experimental shot and who got the placebo among the thousands of volunters. It’s by design, the two (vaccine and placebo) look the same, and only a select group of trial administrators knew who got what. It’s a fascinating facet of clinical trials.
In the first read out of the initial trial results (revealed on November 9, 2020, based on 94 confirmed COVID-19 cases among the volunteers), Pfizer Inc. and BioNTech SE revealed a protection rate of more than 90% against the disease. Moderna’s results, with 94.5% effiicacy based the first 95 confirmed COVID cases among 30,000 volunteers, came a week later. Both results are highly impressive, even for infectious disease experts like Dr Paul Offit, one of the inventors of the rotavirus vaccine.
Now, with mRNA shots approved for the first time, specifically against SARS-CoV-2, the world has a crucial new tool. And that same tool can be used to respond quickly to diseases. For a quick 14-minute explainer on mRNA, view this TedX video:
Full circle
The first vaccine, against smallpox, was made in London in 1796 by Dr Edward Jenner, the father of vaccines. The latest vaccine, against COVID-19, was approved in London. The world of vaccinology has come full circle.
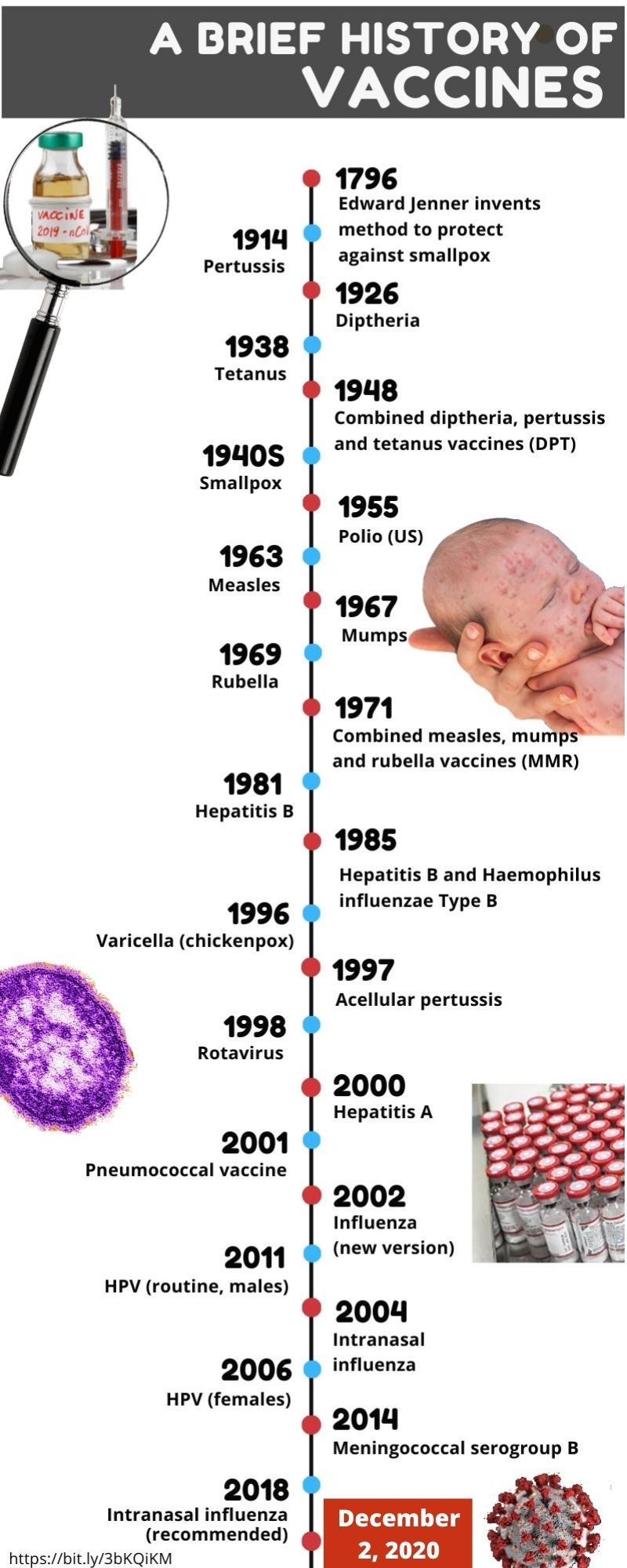
Image Credit: Jay Hilotin / Gulf News


