Dubai: A new COVID-19 test kit that can be self administered at home has been approved by the US Food and Drug Administraition (FDA). What’s more, it can done by anyone 14 and older, with results displayed in 30 minutes. The new test kit utilises a “molecular amplification” technique, known as “reverse transcription loop-mediated isothermal amplification” (RT-LAMP). It is dubbed as a simpler, faster, and less complicated alternative to reverse transcription quantitative polymerase chain reaction (RT-qPCR, os simply just “PCR”) technique. It also minimises exposure for others.
In the US, the kit will be available by doctor’s prescription only: Call your doctor, request the at-home test and it will sent to you. Will it make long wait times, curling lines at COVID test centres a thing of the past? On this question, the jury is still out.
LAMP is quite similar to PCR-based test used extensively today as the most reliable way to detect of SARS-CoV-2 RNA in individuals with known or suspected COVID-19.
There are a number of similarities between this LAMP test and a PCR test. First: Like the PCR test, the rapid test (RT-LAMP) also uses a nasal (pharyngeal) swab, which is then stirred into a sample vial. Second: If someone tests positive, it would then kick up information that the person may be infectious and could therefore take immediate precautions. Third: Both are highly accurate, though LAMP is less accurate than PCR (more on this below).
RT-LAMP: Results within minutes
But there are important differences, or advantages, for LAMP. First: Unlike PCR tests, this newly-approved molecular test can be done at home, or at a patient’s bedside. Second: it switches from diagnosing infections to determining whether someone is infectious — and with results out within minutes, instead of days. Third: It can be more rapidly deployed. Fourth: it’s also potentially less expensive — currently estimated at $50 per test, according to the manufacturer. Fifth: users 14 years and older can be trained with simple instructions tp run the test.
However, there are massive challenges up ahead: While priority should be given for its deployment following licensing, it’s a huge manufacturing and logistical hurdle to make this happen soon.
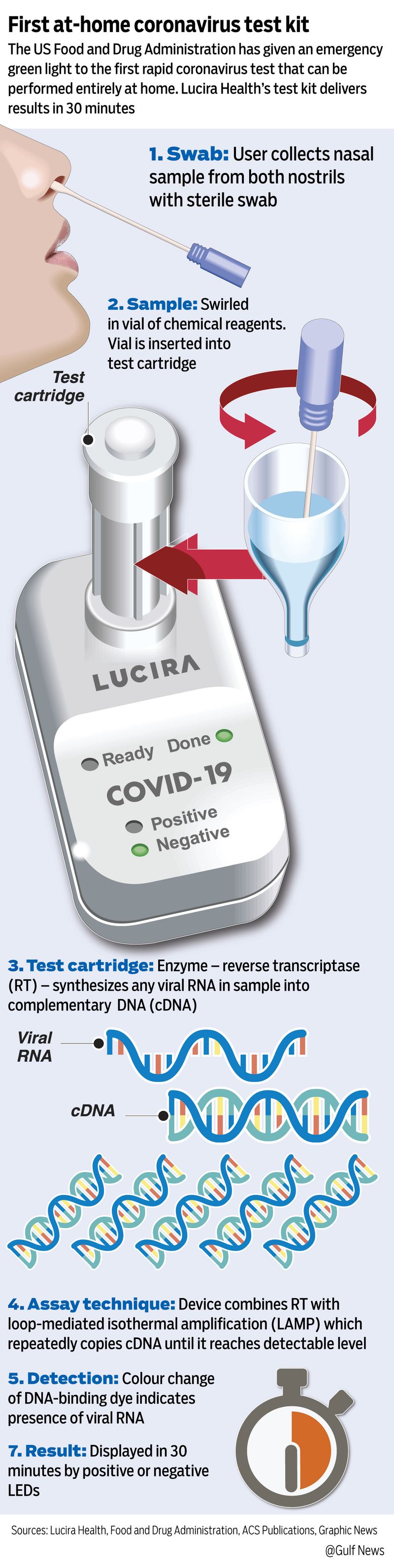
What was approved and when?
On Tuesday, November 17, 2020, the FDA gave an emergency-use approval (EUA) for Lucira Health’s All-in-One COVID-19 test kit, as the first rapid at-home coronavirus test. The kit, manufactured by California-based Lucira Health, delivers results in 30 minutes, the FDA said in statement.
There’s a difference between at-home sample collection and at-home testing. While the FDA has cleared other tests for at-home collection of samples — and laboratories then process these samples — Lucira’s kit takes COVID-19 testing to a higher level. It removes the need for complex lab tests that look for the SARS-CoV-2’s genetic material using the PCR technique.
What’s the basis for emergency-use authorisation?
The US Department of Health and Human Services (HHS) has stated that given the public health emergency posed by COVID-19, it declared that circumstances exist justifying the authorisation of emergency use of “in-vitro” diagnostics for detection and/or diagnosis of the virus that causes COVID-19 subject to the terms of any authorization issued under Section 564(a) of the US Federal Food, Drug, and Cosmetic Act.
What is the technology behind it? How does it work?
Lucira’s at-home test relies on similar principles as PCR by using the LAMP method. Like PCR, LAMP repeatedly copies genetic material until it reaches detectable levels, making it possible to identify the virus even when it is present at only low levels in the respiratory tract.
A typical LAMP reaction mix contains 6 primers which target 8 regions on the bacterial or viral genome. (Source: Science Translational Medicine)
Users taking the test swirl a swab in both of their nostrils, then dip and stir the swab into a vial of chemical reagents. That vial is inserted into a test cartridge to processes the sample.
Positive / Negative
Within half an hour, the test cartridge will light up as “positive” or “negative.”
How accurate is LAMP compared to PCR test?
While faster and less cumbersome than PCR, LAMP is less accurate, according to the FDA. Federal guidelines note that people taking the test should report the results to their health care providers, who must then inform public health authorities to help track the virus’s spread.

According to Lucira, the LAMP test can accurately detect 94 per cent of the infections (true positive) found by the gold-standard PCR-based test. It also correctly identified 98 per cent of the healthy, uninfected people (true negative).

What do other scientists say about RT-LAMP test’s accuracy?
On August 12, 2020, the Science Translational Medicine journal published a study in which they tested a two-colour RT-LAMP assay protocol for detecting SARS-CoV-2 viral RNA using a primer set specific for the N gene.
The team that did the test was led by Viet Loian Dao Thi, of the Schaller Research Groups, Department of Infectious Diseases, Virology, Heidelberg University, Heidelberg, Germany. The RT-LAMP assay was tested on surplus RNA samples isolated from 768 pharyngeal swab specimens collected from individuals being tested for COVID-19. With it, they determined the sensitivity and specificity of the RT-LAMP assay for detecting SARS-CoV-2 viral RNA.
94
%
accuracy of LAMP in detecting infections found by PCR test (94% true positive, and 6% false negative); It also correctly identifies 98% of healthy, uninfected people (2% false positive).
What they found: Compared to an RT-qPCR assay using a sensitive primer set, the STM test found that the RT-LAMP assay reliably detected SARS-CoV-2 RNA with an RT-qPCR cycle threshold (CT) number of up to 30, with a sensitivity of 97.5% and a specificity of 99.7%. Moreover, the researchers also pointed to a newly-developed a swab–to–RT-LAMP assay that did not require a prior RNA isolation step, which retained excellent specificity (99.5%) but showed lower sensitivity (86% for CT < 30) than the RT-LAMP assay. In addition, they also developed a multiplexed sequencing protocol (LAMP-sequencing) as a diagnostic validation procedure to detect and record the outcome of RT-LAMP reactions.
Who is behind Lucira Health?
Lucira Health is a biotech company based in Emeryville, California. It specialises in disposable molecular testing systems and is headed by Erik Engelson, its President & CEO at Lucira Health.
How much is a test?
Lucira said it expects the test to cost $50 (about Dh185) or less.
Will it change the landscape of battle against COVID?
Rapid, reliable tests for detecting existing SARS-CoV-2 infections and assessing virus spread are critical in tackling coronavirus, and helping economies move forward. Approaches to detect viral RNA based on RT-LAMP have potential as simple, scalable, and broadly applicable testing methods could be crucial in the COVID fight. Compared to RT-qPCR–based methods, RT-LAMP assays require incubation at a constant temperature, therefore eliminating the need for sophisticated instrumentation.
The Lurica COVID-19 test kit approved by the FDA is a molecular single-use test that is intended to deted the SARS-CoV-2. It is authorised for prescription home use with self-collected nasa swap samples in people aged 14+ who are suspected of having COVID-19 by a healthcare provider.
Image Credit: Lucira Health
In the past, a number of COVID-19 diagnostic tests authorised for at-home collection of samples, but not for processing those samples. “This is the first that can be fully self-administered and provide results at home,” explained FDA Commissioner Stephen M Hahn, M.D. “This new testing option is an important diagnotic advancement to address the pandemic and reduce the public burden of disease transmission,” he added.
Research on accuracy of LAMP testing
On August 12, 2020 Science Translational Medicine journal published a study led by Viet Loian Dao Thi of the Department of Infectious Diseases, Virology, Heidelberg University, Heidelberg, Germany. The researchers tested a two-colour RT-LAMP assay protocol for detecting SARS-CoV-2 viral RNA using a primer set specific for the N gene.
The RT-LAMP assay was tested on surplus RNA samples isolated from 768 pharyngeal swab specimens collected from individuals being tested for COVID-19. With it, they determined the sensitivity and specificity of the RT-LAMP assay for detecting SARS-CoV-2 viral RNA.
What researchers found: Compared to an RT-qPCR assay using a sensitive primer set, the RT-LAMP assay reliably detected SARS-CoV-2 RNA with an RT-qPCR cycle threshold (CT) number of up to 30, “with a sensitivity of 97.5% and a specificity of 99.7%”.
Moreover, the researchers also pointed to a newly-developed a swab–to–RT-LAMP assay that did not require a prior RNA isolation step, which retained specificity (99.5%) but showed lower sensitivity (86% for CT < 30) than the RT-LAMP assay. They also developed a multiplexed sequencing protocol (LAMP-sequencing) as a diagnostic validation procedure to detect and record the outcome of RT-LAMP reactions.
When will it be available?
Scaling up production fast enough is a key challenge. Lucera Health said they will release the kits in Florida and California first. Wider distribution is slated for early Spring 2021, according to US media reports. The rest of the world may have to wait for something similar or better. Once released, though, experts believe it will have an impact in the fight against COVID-19.

